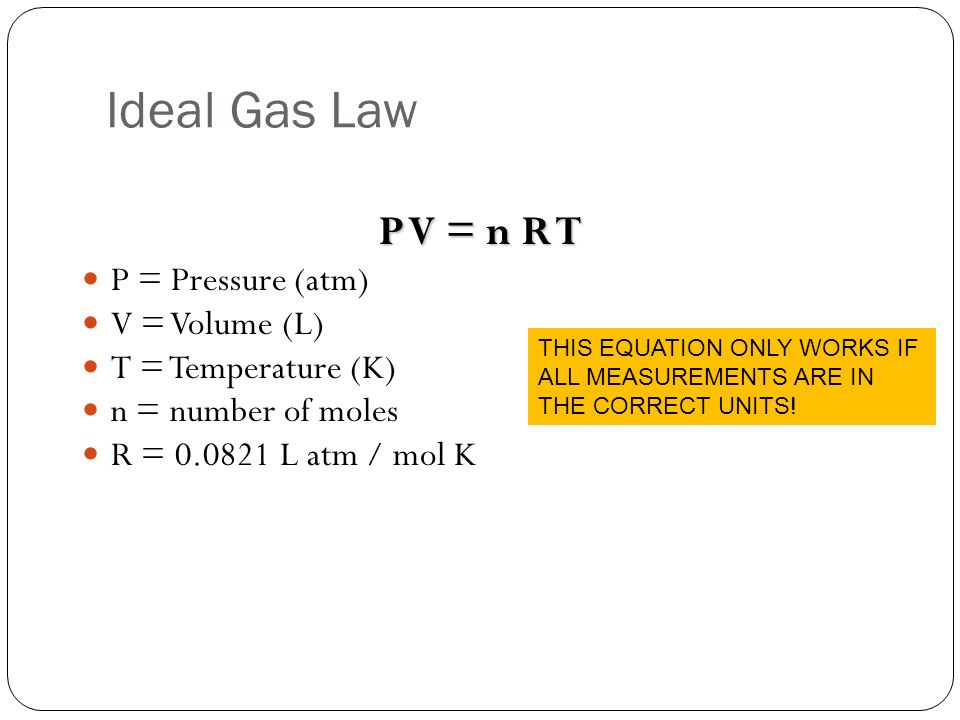
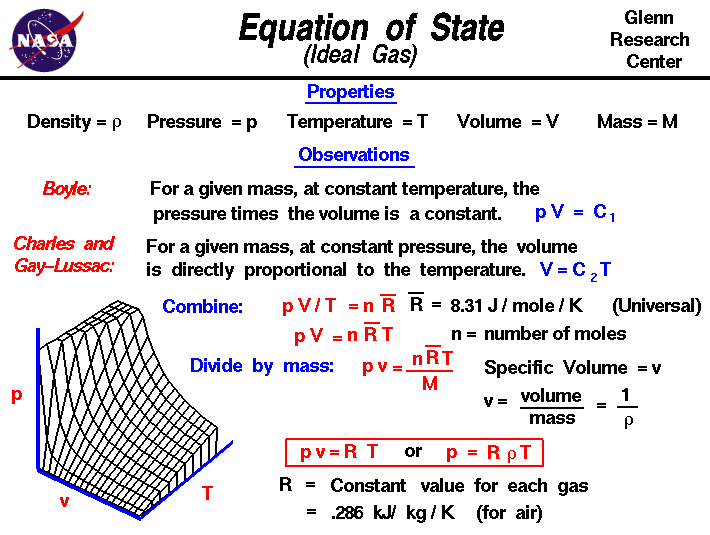
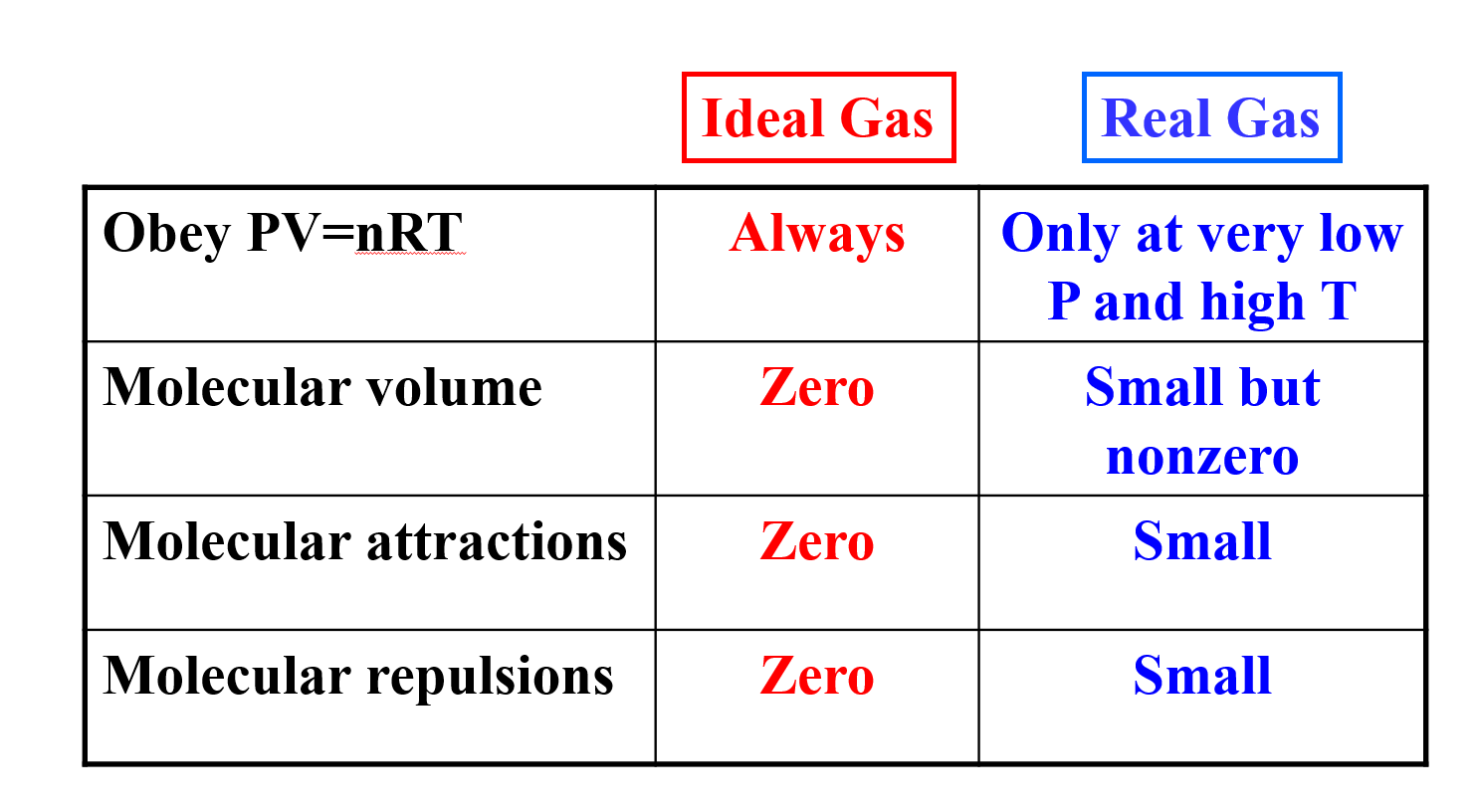
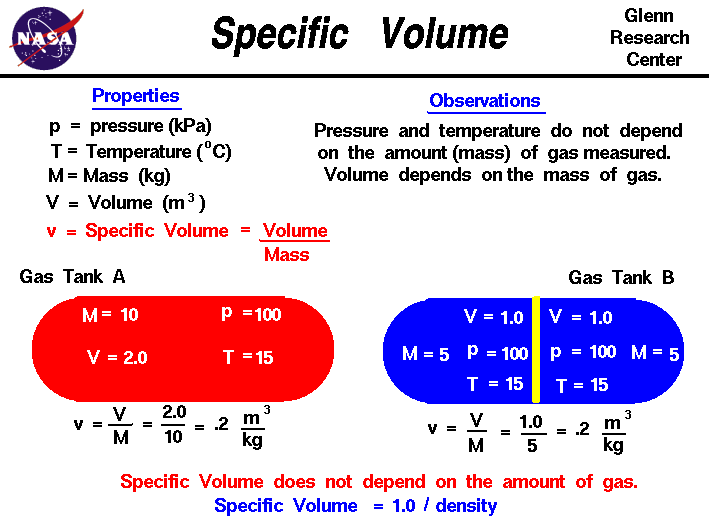
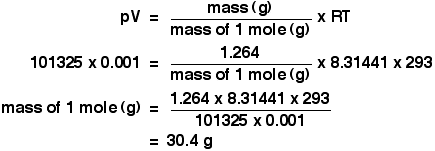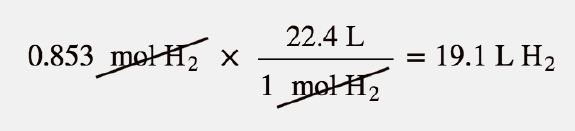molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises
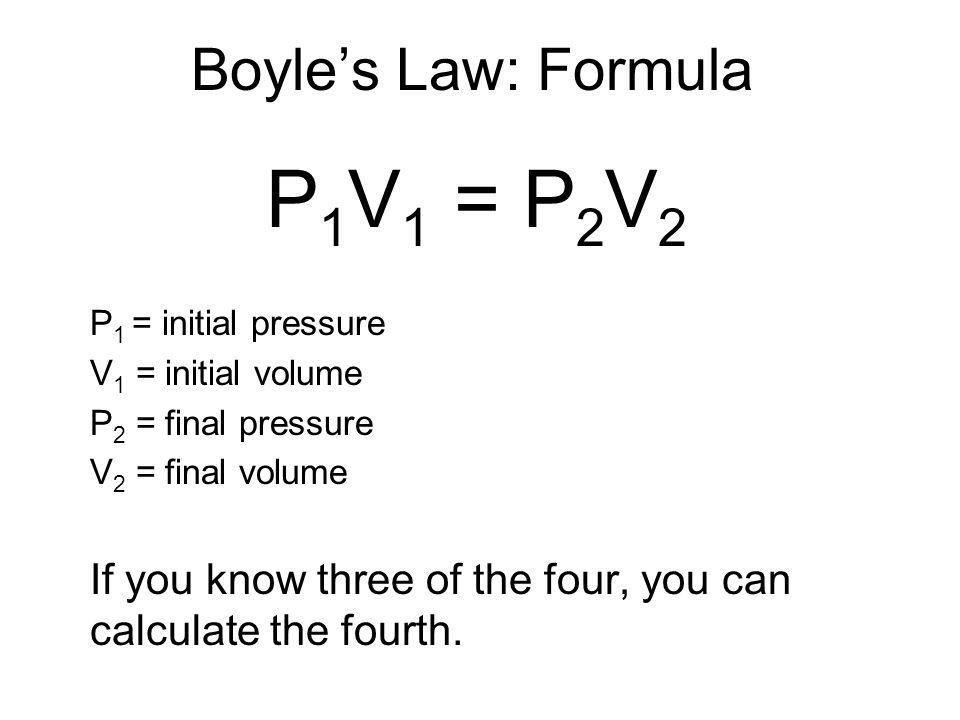
A "1.32 L" volume of gas has a pressure of "1.00 atm". What will the volume be if the pressure is increased to "30.0 atm"? | Socratic