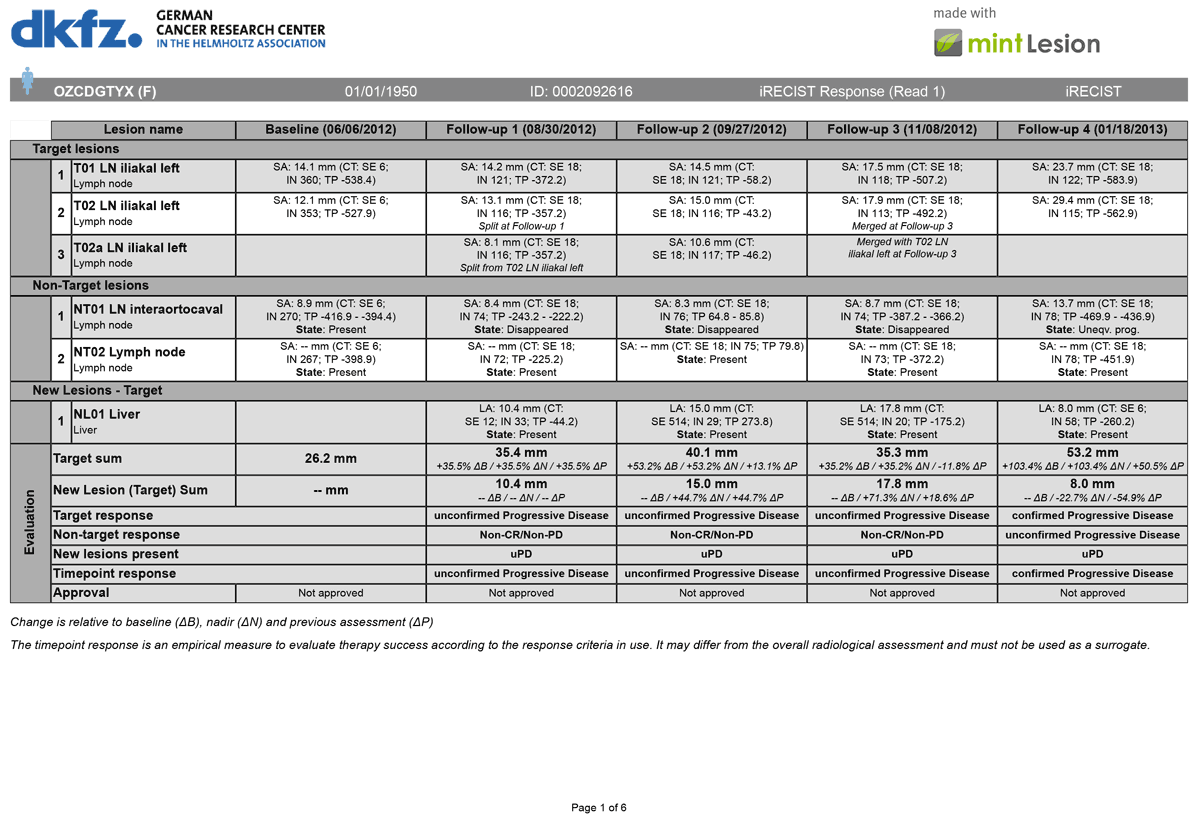
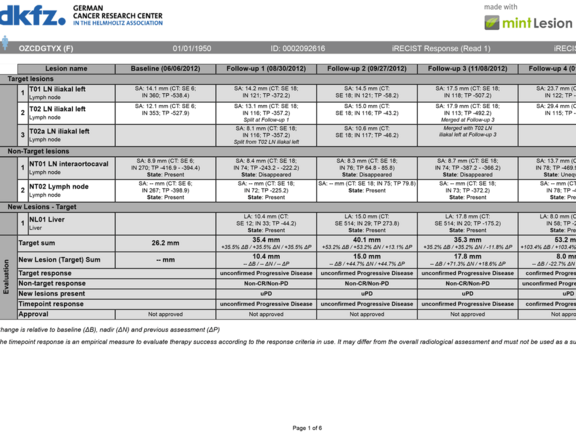
Comparison of Radiological Tumor Response Based on iRECIST and RECIST 1.1 in Metastatic Clear-Cell Renal Cell Carcinoma Patient
Comparison of Radiological Tumor Response Based on iRECIST and RECIST 1.1 in Metastatic Clear-Cell Renal Cell Carcinoma Patient

PDF) Comparison of RECIST 1.1 and iRECIST in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis

Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: pooled FDA analysis | Journal for ImmunoTherapy of Cancer

Kaplan-Meier curves of OS and PFS based on iRECIST to compare outcomes... | Download Scientific Diagram

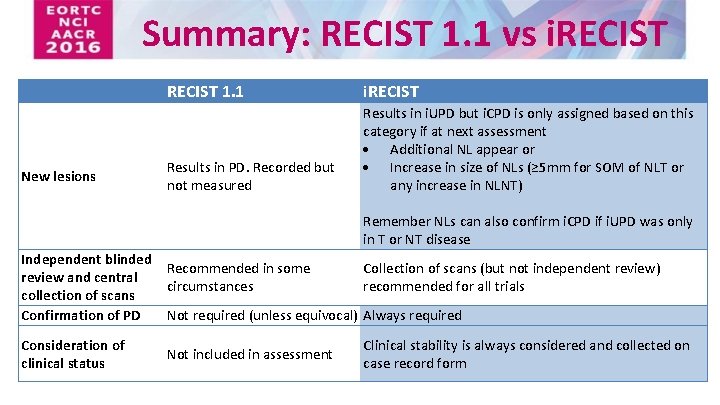
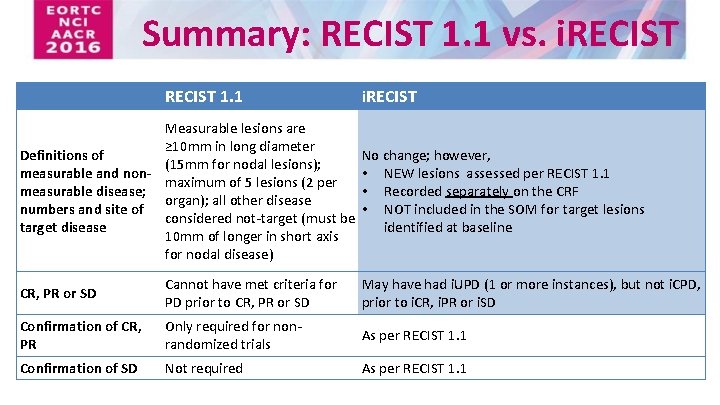
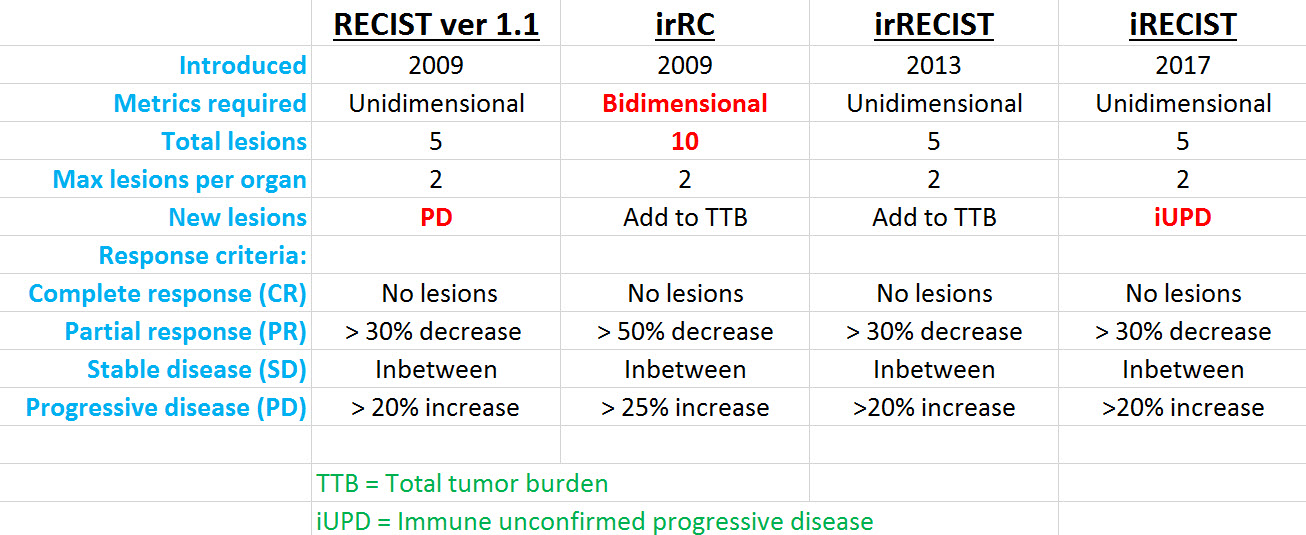
iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics - The Lancet Oncology
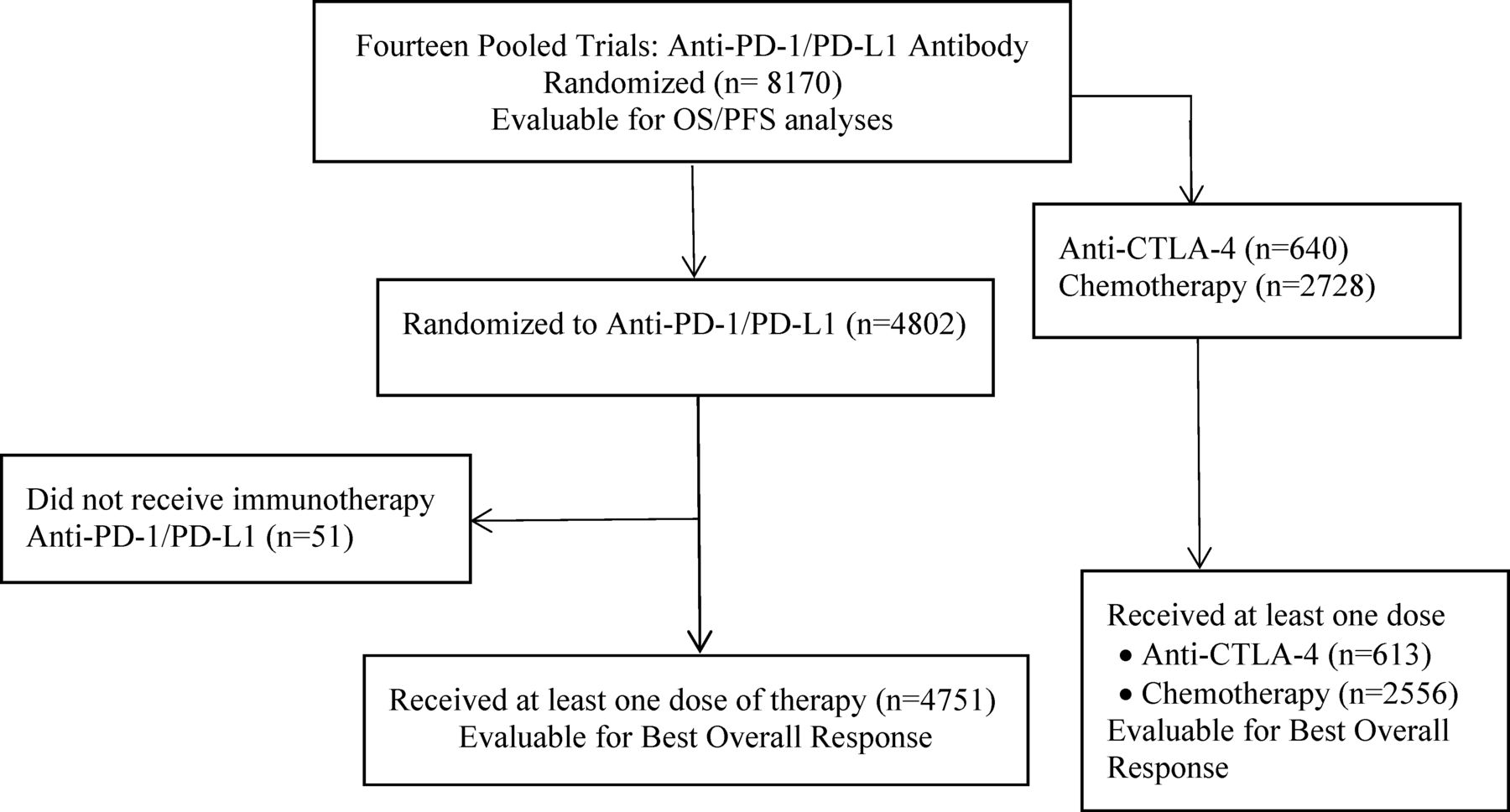
Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: pooled FDA analysis | Journal for ImmunoTherapy of Cancer

iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics - The Lancet Oncology

iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. - Abstract - Europe PMC

iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. - Abstract - Europe PMC

Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: pooled FDA analysis | Journal for ImmunoTherapy of Cancer
Simplifying the Derivation of Best Overall Response per RECIST 1.1 and iRECIST in Solid Tumor Clinical Studies

PDF) Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: pooled FDA analysis

Response Criteria for Intratumoral Immunotherapy in Solid Tumors: itRECIST | Journal of Clinical Oncology