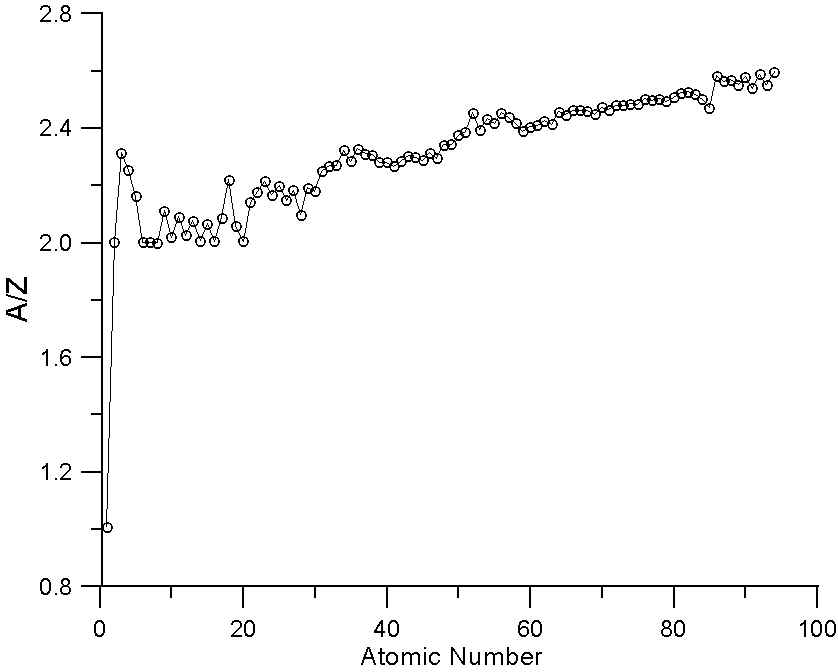
Atomic number Z versus mass-over-charge ratio A=Z of those ions which... | Download Scientific Diagram

How To Calculate Relative Atomic Mass - What is Relative Mass? How to Calculate Relative Atomic Mass along with FAQs

A radioactive nucleus (initial mass number A and atomic number Z) emits 3 alpha - particles and 2 positrons. The ratio of number of neutrons to that of protons in the final
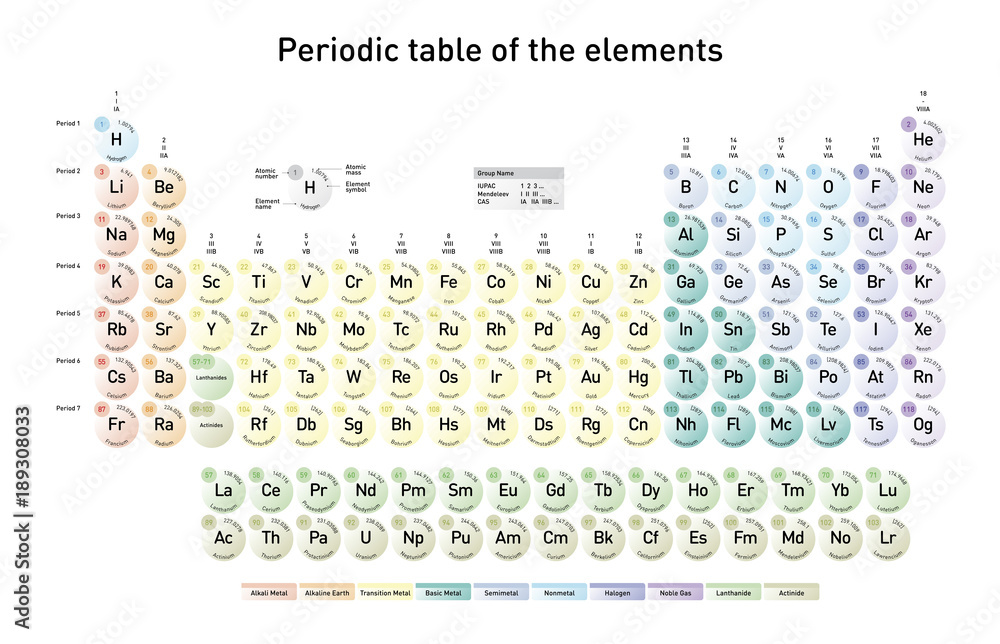
Modern Periodic Table of the Elements with atomic number, element name, element symbol and atomic mass, in english language Stock Vector | Adobe Stock

SOLVED: For lighter, stable isotopes, the ratio of the mass number to the atomic number is close to a certain value. What is the value? What happens to the value of the

Indium has atomic number 49 and atomic mass 114.8 g. contains a mixture of indium-112 and indium-115

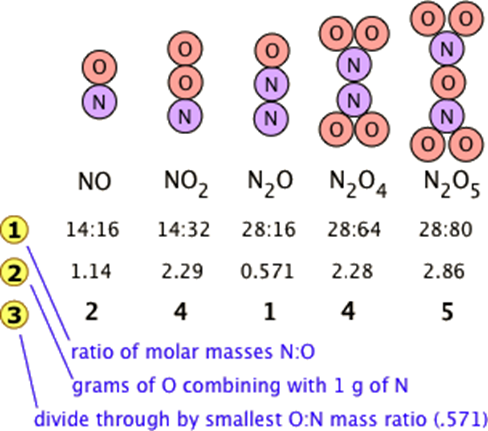
Find the ratio by mass of combining element in the following compounds. a) CO2 b) NH3 (If C = 12 u, O - 16 u, and H = 1 u)

Atomic Ratios in Compounds | How to Find the Number of Atoms in a Compound - Video & Lesson Transcript | Study.com

SOLVED: An isotope with atomic number 64 and mass number 158 is found to have a mass ratio relative to that of carbon-12 of 13.16034. What is the isotope, what is its

The atomic mass of lead is 208 and its atomic number is 82. The atomic mass of bismuth is 209 and its atomic number is 83. The ratio of neutrons/protons in the atom



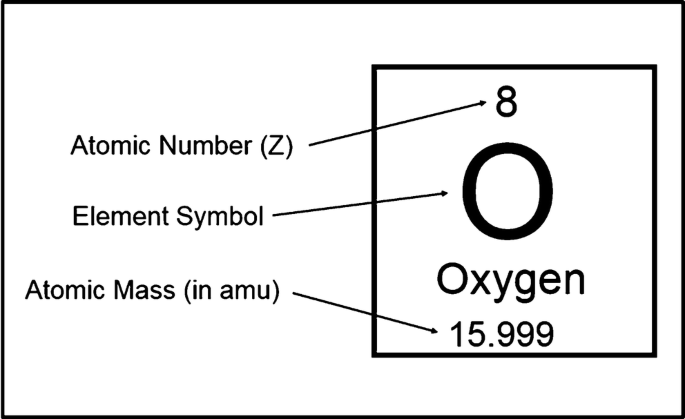
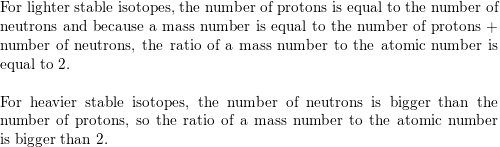


:max_bytes(150000):strip_icc()/boron-illustration-545864379-5838819f5f9b58d5b1c57b5f.jpg)



