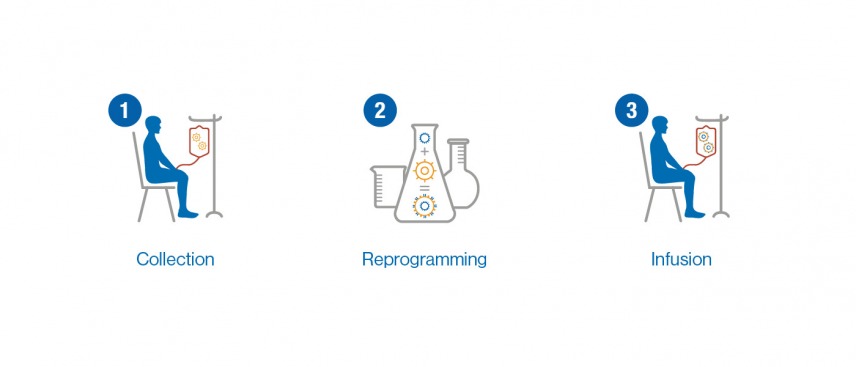
Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
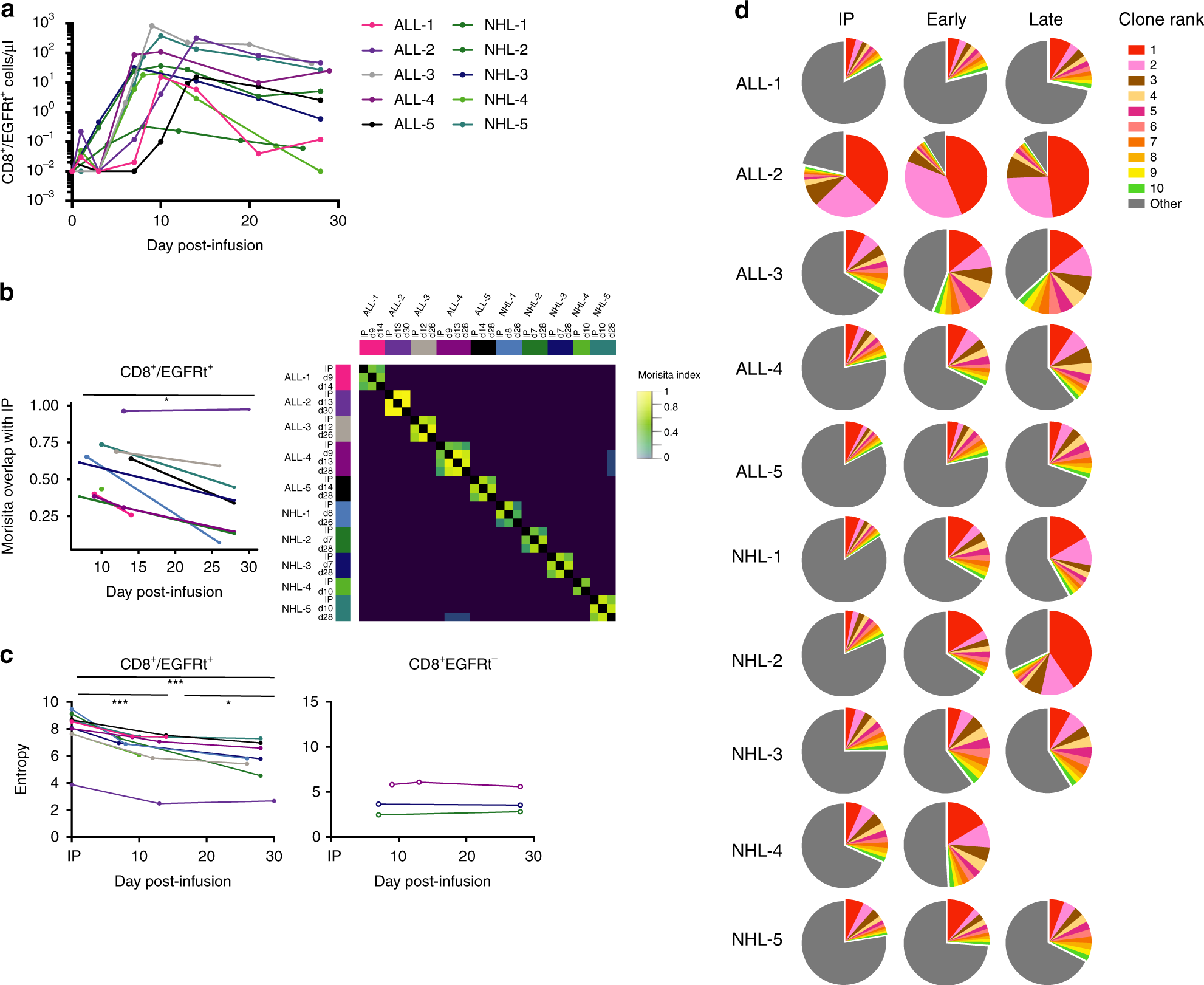
Clonal kinetics and single-cell transcriptional profiling of CAR-T cells in patients undergoing CD19 CAR-T immunotherapy | Nature Communications
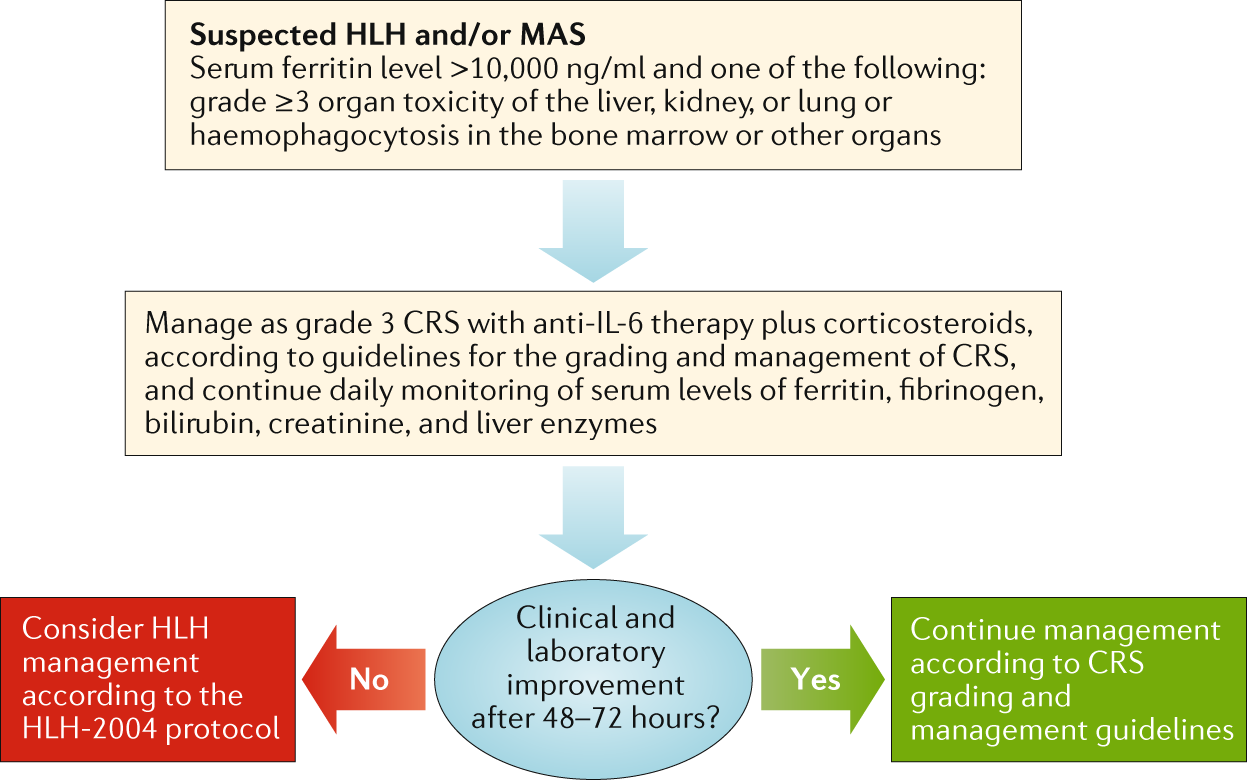
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology
Novartis fills manufacturing gap for CAR-T therapy Kymriah with first Asian production facility | Fierce Pharma

CAR-T Cell Product Development Guidance Covers Previous Recipients, 'Bridging Therapy' :: Pink Sheet
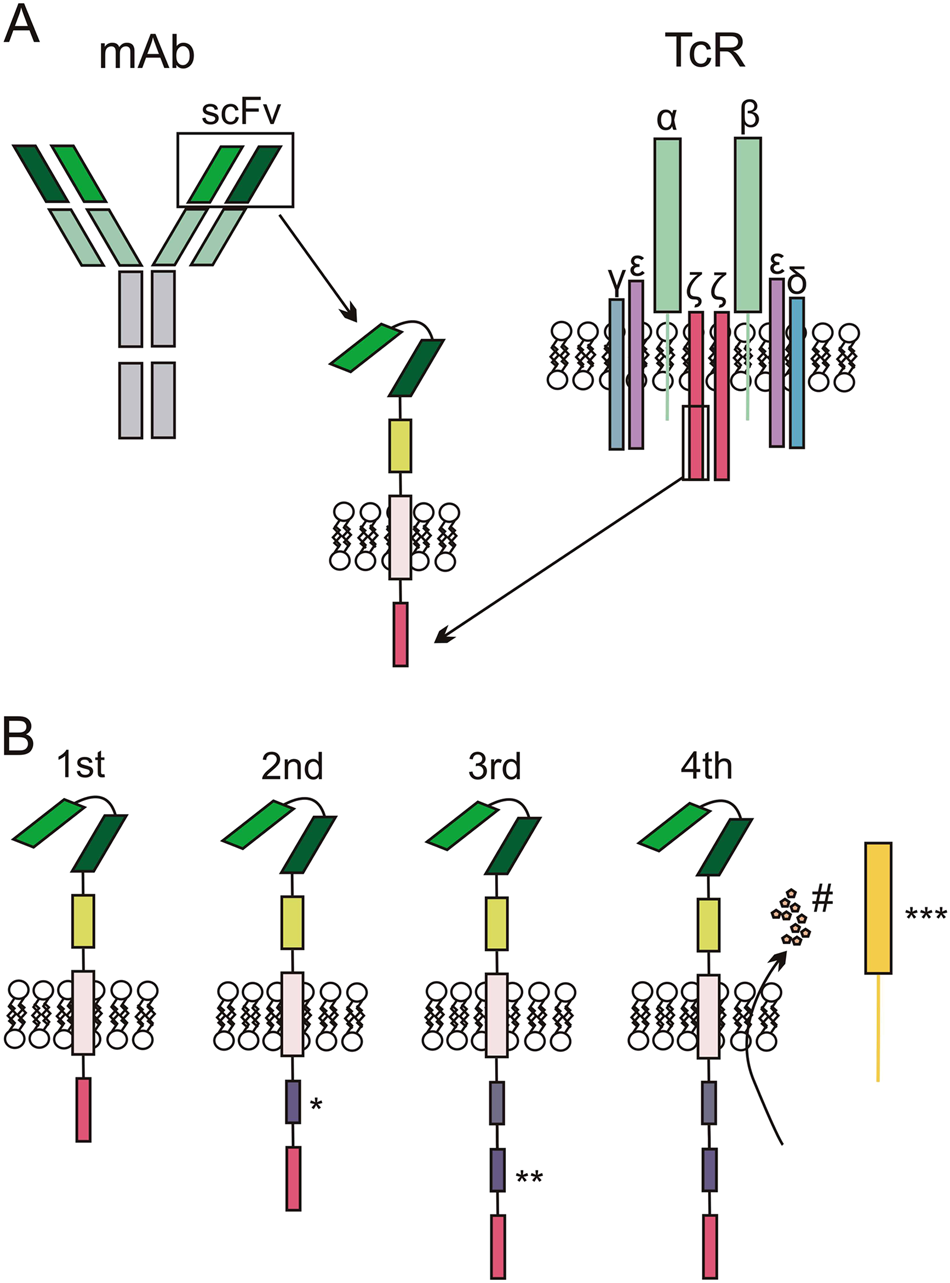
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar

PDF) Chimeric Antigen Receptor-T Cell Therapy: Practical Considerations for Implementation in Europe

The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19 - Cytotherapy

Optimizing CAR-T Cell Manufacturing Processes during Pivotal Clinical Trials: Molecular Therapy - Methods & Clinical Development

Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma | Nature Communications
FDA committee takes on complex gene therapy safety questions with Novartis' Zolgensma providing lessons learned | Fierce Biotech
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar